How Does a CR2032 Battery Work?
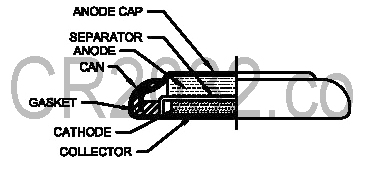 CR2032 batteries are no different from other kinds of batteries in terms their function. Each battery is a tiny self-contained electrochemical cell with a positive and negative end. The CR2032 battery uses a combination of lithium and manganese dioxide to power a chemical reaction that converts chemical energy into the electrical energy that powers portable devices like watches and remote controls. CR2032 batteries are no different from other kinds of batteries in terms their function. Each battery is a tiny self-contained electrochemical cell with a positive and negative end. The CR2032 battery uses a combination of lithium and manganese dioxide to power a chemical reaction that converts chemical energy into the electrical energy that powers portable devices like watches and remote controls.
Every battery has a chemical it uses for the positive end and another it uses for the negative end. In the CR2032, the positive electrode is composed of manganese dioxide while the negative electrode is made of lithium. The lithium anode is placed on top of the manganese dioxide cathode, and a salt bridge separator is placed between them.
In this redox reaction, the lithium side oxidizes, and the manganese dioxide side reduces. As this occurs, electricity flows through the devices that the CR2032 battery powers. More lithium comes out of solution and builds up on the surface of the negative end while more manganese dioxide goes into solution.
When the chemical reaction is finished, the electrical current ceases to flow through the CR2032 battery. At this point, most CR2032 batteries cannot be recharged. They must be disposed of properly.
The lithium in the CR2032 is what gives the battery its power. Very few small sized batteries can deliver the same punch. That’s another reason why the CR2032 is not rechargeable. If it were, it would discharge itself too quickly, and it wouldn’t be nearly as practical for the watches and remote controls it typically powers. |